Percutaneous Arteriovenous Fistula Creation with the WavelinQ 4-French EndoAVF System: A Single-Center Retrospective Analysis of 30 Patients
Clinical question
To assess the safety and efficacy of percutaneous arteriovenous fistula (pAVF) creation with WavelinQ 4F EndoAVF System.
Take away point
The WavelinQ 4F EndoAVF is a safe and effective system to create pAVFs with high maturation rate and long-term patency.
Reference
Kitrou PM, Balta L, Papachristou E, Papasotiriou M, Katsanos K, Theofanis M, Papadoulas S, Anagnostopoulos F, Georgopoulou GA, Goumenos D, Karnabatidis D. Percutaneous Arteriovenous Fistula Creation with the WavelinQ 4-French EndoAVF System: A Single-Center Retrospective Analysis of 30 Patients. J Vasc Interv Radiol. 2022 Jan;33(1):33-40. doi: 10.1016/j.jvir.2021.09.021.
Click here for abstract
Study design
Single-center retrospective analysis with 30 patients requiring pAVFs for long-term hemodialysis.
Funding Source
The authors of this study receive consultancy fees and lecture honoraria from BD.
Setting
Academic setting. Patras University Hospital, Patras, Greece.
Figure

Figure 2a and b. Catheter alignment and different access options (only parallel approach is shown). Images showing a parallel brachial access (brachial artery and vein) of the catheters aiming to create a percutaneous arteriovenous fistula at the ulnar side (a, b). The images show the distance between the electrode (arrow) and the ceramic backstop before (a) and after (b) activation. The distance between the catheters in image (a) reflects the walls of the vessels and the tissue between the vessels. Following activation and anastomosis creation, the electrode is in contact with the ceramic backstop. In the image (a), the square orientation of the indicators at the front tip of the catheters is shown (arrowhead).
Study design
Single-center retrospective analysis with 30 patients requiring pAVFs for long-term hemodialysis.
Funding Source
The authors of this study receive consultancy fees and lecture honoraria from BD.
Setting
Academic setting. Patras University Hospital, Patras, Greece.
Figure

Figure 2a and b. Catheter alignment and different access options (only parallel approach is shown). Images showing a parallel brachial access (brachial artery and vein) of the catheters aiming to create a percutaneous arteriovenous fistula at the ulnar side (a, b). The images show the distance between the electrode (arrow) and the ceramic backstop before (a) and after (b) activation. The distance between the catheters in image (a) reflects the walls of the vessels and the tissue between the vessels. Following activation and anastomosis creation, the electrode is in contact with the ceramic backstop. In the image (a), the square orientation of the indicators at the front tip of the catheters is shown (arrowhead).
Figure 3. Final angiogram of a procedure performed with brachial arterial access. Contrast was injected from the brachial artery. The different parts of the newly formed vascular access circuit are pointed out. 1, brachial artery; 2, radial artery; 3, anastomosis; 4, radial vein (with some spasm); 5, perforator vein; 6, cephalic vein; and 7, basilic vein. The ulnar artery (A), main ulnar artery (B), and interosseous artery (C) outside the vascular access circuit are also apparent.
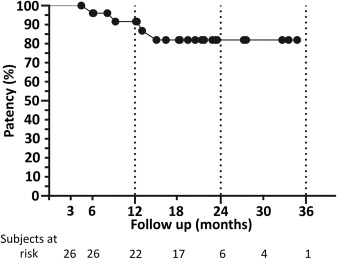
Figure 4. Kaplan-Meier survival curve of the 26 cases that matured and achieved cannulation with subjects at risk.
Post Author
Brian Stephen Wong, MD MPH
PGY-3 Diagnostic Radiology Resident
Summary
Percutaneous arteriovenous fistulas (pAVFs) are a new endovascular technique to create hemodialysis access for patients with chronic kidney failure. Two systems are currently available: the WavelinQ EndoAVF and the Ellipsys Vascular Access System. The WavelinQ creates an anastomosis between the radial and ulnar vessels, while the Ellipsys System uses the perforating vein and proximal radial artery. pAVFs offer an additional technique for long-term hemodialysis access and may have similar outcomes with surgical arteriovenous fistula, however limited data has been published about the safety and efficacy.
Thirty patients met the inclusion criteria of being >18 years old, Stage V CKD, Target A/V diameters >2.5mm with perforating forearm vein of >2mm. Patient with active hypercoagulable state, infection, extensive vascular calcification, and contrast allergy were excluded from the study The study was a single-center, single-arm retrospective study of arteriovenous fistulas created by the 4Fr WavelinQ system.
This study is one of the first to address the safety and efficacy of pAVFs. One distinct advantage of pAVFs is that the procedure does not compromise the creation of future surgical AVFs at the same site. While this technique is not risk-free, it appears that it is a safe alternative for providing hemodialysis access to patients with CKD. Other studies with the WavelinQ EndoAVF system have validated the high success rate and efficacy:
Thirty patients met the inclusion criteria of being >18 years old, Stage V CKD, Target A/V diameters >2.5mm with perforating forearm vein of >2mm. Patient with active hypercoagulable state, infection, extensive vascular calcification, and contrast allergy were excluded from the study The study was a single-center, single-arm retrospective study of arteriovenous fistulas created by the 4Fr WavelinQ system.
- 100% (30/30) patients had technical success in pAVF creation.
- In thirty patients that underwent pAVF creation, the cannulation rate was 87% at mid-term follow-up time (mean f/u time 547 +/- 315 days).
- 16 reinterventions (i.e. coil embolization or angioplasty) were necessary to facilitate cannulation without need for open surgical procedures.
- 7 maintenance procedures were performed in 6 of 27 matured pAVFs.
- 22 out of 33 (73%) pAVFs were non-thrombosed and cannulated at the end of the study.
- Relatively safe with adverse event rate (i.e. brachial artery pseudoaneurysm) in 2/30 patients (6.7%).
Commentary
This study is one of the first to address the safety and efficacy of pAVFs. One distinct advantage of pAVFs is that the procedure does not compromise the creation of future surgical AVFs at the same site. While this technique is not risk-free, it appears that it is a safe alternative for providing hemodialysis access to patients with CKD. Other studies with the WavelinQ EndoAVF system have validated the high success rate and efficacy:
- Endovascular Access System Enhancements study (Berland et al. 2019; 4Fr) was the first to evaluate the current 4Fr system and reported 100% technical success in 32 patients with both ulnar and radial AVFs.
- The FLEX study (Rajan et al. 2015; 6Fr) enrolled 33 pts with a success rate of 97% and one adverse effect (brachial pseudoaneurysm).
- The Novel Endovascular Access Trial (Lok et al. 2017; 6Fr) with 60 patients demonstrated a high success rate of 98% with only 2% adverse effects in ulnar pAVFs.
- Zemela et al. 2021 (6Fr) with 35 patients reported technical success rate of 100% and one pseudoaneurysm.
- Radosa et al. 2017 (6Fr) with 8 patients reported technical success rate of 100%.
A recent study by Inston et al. compared pAVRs (with both generations of the WavelinQ system) and surgical radiocephalic AVFs and reported comparable patency rates. A study by Shahverdyan demonstrated that WavelinQ and Ellipsys are no different in outcome. A Multicenter European/Canadian discovered that coil embolization at index reduced further need to treat.
Similar to other studies on novel techniques of percutaneous arteriovenous fistula creation, this study's clinical value remains uncertain and practice adoption of the studied devices/techniques difficult given the small sample size, selection bias, inconsistent follow-up duration, procedural and fistula metrics incompleteness, and lack of prospective control arm(s). The authors rightfully described their findings as early experience, limited in scope, and will require external larger prospective clinical trials for validation.
Post Author
Brian Stephen Wong, MD MPH
PGY-3 Diagnostic Radiology Resident
University of Texas Medical Branch
Edited and formatted by @NingchengLi
Interventional Radiology Resident
Dotter Institute, Oregon Health and Science University